Abstract
In this study, we sought to develop Manganese Ferrite (MnFe2O4) based Magnetic Nano-cluster (MNC) for specifically designed for the detection of triple-negative breast cancer (TNBC) through the use of magnetic resonance imaging (MRI). The MnFe2O4-based MNCs were synthesized utilizing a thermal decomposition method, which allowed for precise control over their structural properties. Following synthesis, the average size of the MNC was measured to be 69 ± 27.4 nm, a dimension that is optimal for effective imaging and cellular interactions. To evaluate the biocompatibility of the synthesized MNC, we conducted a series of cytotoxicity tests. These tests revealed that cellular viability remained above 82 % across all treatment concentrations, indicating that the MNCs exhibit minimal cytotoxic effects even after intravenous injection. This finding is particularly significant as it underscores the potential safety of using MNC for in vivo applications. Subsequently, the MNCs were administered intravenously into the tail vein of nude mice, and MRI imaging was performed at various time intervals to monitor their distribution and behavior within the biological system. The results demonstrated a notable enhancement of contrast in the imaging of the xenograft mouse model, which utilized MNC for visualization. This enhancement is indicative of the MNC’s efficacy in highlighting tumor sites, thus facilitating improved diagnostic capabilities. The implications of these findings are substantial, as they not only contribute to the advancement of imaging technologies but also pave the way for future clinical applications of MNC in oncology. We anticipate that these results will serve as a valuable foundation for ongoing research and development, ultimately leading to the establishment of MNC as a reliable diagnostic tool for patients diagnosed with TNBC, a particularly aggressive and challenging subtype of breast cancer.
1. Introduction
Manganese ferrite (MnFe₂O₄), a mixed metal oxide, has garnered considerable attention in recent years due to its distinctive amalgamation of magnetic, chemical, and physical properties [1]. This material exhibits robust ferromagnetism, rendering it suitable for applications in magnetic storage devices and biomedical imaging. Its elevated Curie temperature enables MnFe₂O₄ to maintain its magnetic characteristics even at high temperatures, which is advantageous for high-performance applications. Furthermore, the chemical stability of manganese ferrite ensures resistance to oxidation and corrosion, thereby enhancing its durability across diverse environments [2,3]. The biocompatibility of MnFe₂O₄ further broadens its potential in biomedical applications, including drug delivery systems and hyperthermia treatment. Additionally, its catalytic efficacy in the degradation of organic pollutants positions it as a valuable material for environmental remediation [4]. The cost-effectiveness of MnFe₂O₄, attributed to the abundance of its raw materials, renders it an attractive option for industrial applications. Moreover, its versatility in synthesis methods allows for the customization of properties to meet specific application requirements [5]. In the realm of medical imaging, manganese ferrite (MnFe₂O₄) has emerged as a promising contrast agent in T2-weighted magnetic resonance imaging (MRI) due to its unique magnetic properties and biocompatibility [6]. The high magnetic susceptibility of MnFe₂O₄ enhances the relaxation times of adjacent protons, resulting in a significant increase in signal contrast in T2-weighted images. This characteristic is particularly advantageous for visualizing tumors and other pathological tissues, where improved contrast can facilitate early diagnosis and more effective treatment planning. Furthermore, the ability to tailor the size and surface characteristics of MnFe₂O₄ nanoparticles allows for optimized biodistribution and reduced toxicity, making them suitable for in vivo applications [7]. The stability of MnFe₂O₄ under physiological conditions further supports its use as a reliable contrast agent, minimizing the risk of adverse reactions. Additionally, the potential for functionalization of MnFe₂O₄ with targeting ligands opens new avenues for the specific imaging of disease markers, thereby enhancing the precision of diagnostic imaging. The cost-effectiveness of manganese ferrite, combined with its favorable magnetic properties, positions it as a competitive alternative to traditional gadolinium-based contrast agents. The advantages of magnetic nanoclusters based on MnFe₂O₄ compared to magnetic single nanoparticles are substantial. Nanoclusters facilitate enhanced magnetic interactions, thereby improving their magnetic properties. Furthermore, their increased surface area contributes to greater reactivity in various chemical processes. Additionally, they demonstrate superior magnetic stability, rendering them more resilient to fluctuations in external magnetic fields. Their capacity for customization into diverse shapes and sizes provides significant versatility for a range of applications. These attributes collectively augment their potential for utilization in various domains, including biomedicine, catalysis, and energy storage [8].
Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer characterized by the absence of estrogen and progesterone receptors, as well as a lack of overexpression of the human epidermal growth factor receptor 2 (HER2) [9]. This malignancy presents significant challenges in both diagnosis and treatment, often correlating with a poorer prognosis and limited therapeutic options [10]. Recent investigations have explored the utility of T2-weighted magnetic resonance imaging (MRI) as a diagnostic modality for TNBC, aiming to enhance the detection and characterization of tumors. T2-weighted MRI offers several advantages in the assessment of TNBC, including high contrast resolution that facilitates the differentiation of tumor tissue from surrounding healthy breast tissue, which is crucial for accurate tumor delineation [11]. However, the application of T2-weighted MRI in TNBC diagnosis has limitations. Variability in imaging protocols and interpretation can lead to inconsistencies in diagnostic accuracy. Additionally, the presence of fibroglandular tissue in younger women, who are more likely to develop TNBC, complicates the interpretation of MRI findings, potentially resulting in false positives or negatives [12]. Moreover, while T2-weighted MRI provides valuable insights into tumor characteristics [13], it may not be as effective in assessing critical features such as lymph node involvement or distant metastasis, which are essential for staging and treatment planning. And also, it is associated with certain rates of false negatives and false positives. Some of the Research indicates that the false negative rate of MRI in TNBC ranges from approximately 3.2 % to 4.4 % [14]. While specific figures for the false positive rate are not explicitly stated, it may arise due to cognitive errors. This information is instrumental in understanding the limitations of MRI in the diagnosis of TNBC. These statistics suggest a need for ongoing research and improvements to enhance the accuracy of TNBC diagnosis. To address these limitations, there is an increasing need for magnetic nanoclusters, which significantly enhance magnetic moments compared to single nanoparticles, to improve early diagnosis and accuracy.
This study indicates that the application of manganese ferrite (MnFe₂O₄)-based magnetic nanoclusters in the diagnosis of triple-negative breast cancer (TNBC) demonstrates superior effectiveness compared to magnetic single nanoparticles. Magnetic nanoclusters exhibit enhanced magnetic properties and greater surface area, facilitating improved targeting and imaging capabilities. This increased efficiency allows for more accurate detection of TNBC, potentially leading to better patient outcomes. Furthermore, the unique structural characteristics of nanoclusters contribute to their stability and biocompatibility, establishing them as a promising tool in oncological diagnostics. Overall, the advantages of magnetic nanoclusters position them as a more effective alternative for TNBC diagnosis.
2. Experimental Methods
2.1. Materials
N6-Carbobenzyloxy-L-lysine, 4-imidazoleacetic acid, (3-aminopropyl) trimethoxysilane, 1-ethyl-3-(N, N-dimethylaminopropyl) carbodiimide hydrochloride, trifluoroacetic acid, and hydrobromic acid solution (33 wt% in acetic acid) were procured from Sigma-Aldrich. Anhydrous tetrahydrofuran, N, N-dimethylformamide, and dimethylsulfoxide were also obtained from Sigma-Aldrich. A 1 N sodium hydroxide solution, iron (III) acetylacetonate, manganese (II) acetylacetonate, 1,2-hexadecanediol, lauric acid, lauryl amine, benzyl ether, deuterium oxide (D2O), and dimethylsulfoxide-d6 were sourced from the same supplier. Triphosgene was acquired from Tokyo Chemical Industry Co., while 1-hydroxybenzotriazole hydrate was obtained. n-Hexane and diethyl ether were obtained.
2.2. Synthesis of MNC
Initially, monodisperse manganese ferrite (MnFe2O4) pre-MNC were synthesized through the thermal decomposition of metal-organic precursors in the presence of nonpolar organic solvents. Specifically, iron (III) acetylacetonate (2 mmol), manganese (II) acetylacetonate (1 mmol), 1,2-hexadecanediol (10 mmol), lauric acid (6 mmol), and laurylamine (6 mmol) were dissolved in 20 mL of benzyl ether. The resulting solution was preheated to 200 ℃ for 2 hours under an inert nitrogen atmosphere and subsequently refluxed at 300 ℃ for 1 hour. Upon cooling the reaction mixture to room temperature, the products were purified using an excess of pure ethanol. Utilizing the seed-mediated growth method, pre-MNCs approximately 10-11 nm in size were synthesized. Twenty milligrams of the as-synthesized pre-MNC were dissolved in 4 mL of n-hexane and then introduced into 20 mL of deionized water containing 200 mg of poly (lactic acid) (PLA). After the mutual saturation of the organic and aqueous phases, the mixture was subjected to ultrasonication at 190 W for 15 minutes while being vigorously stirred at 1200 rpm, followed by an additional 4 hours of stirring to evaporate the residual hexane. The product was subsequently collected through sequential centrifugation under three distinct conditions (at 15,000 rpm for 30 minutes, at 8,000 rpm for 15 minutes, and at 5,000 rpm for 15 minutes) to minimize the size distribution of the polydispersed MNC.
2.3. Cytotoxicity Test of MNC
The cytotoxicity of the MNC against MDA-MB-231 cells was assessed utilizing a CCK-8 cell proliferation assay kit. MDA-MB-231 cells were plated in a 96-well plate at a density of 2 × 104 cells per well and cultured at 37 ℃ in a humidified environment with 5 % CO2. Following 24 hours of incubation, the cells were exposed to MNC at varying concentrations for an additional 24 hours. To evaluate the effects of MNC, 50 μg/mL MNC were incubated for 5 minutes, and then serially diluted twofold into nine aliquots that were administered to the MDA-MB-231 cells for 24 hours. Subsequently, the cells were treated with CCK-8 solution according to the manufacturer’s protocol. Cell viability was quantified spectrophotometrically at 450 nm using a microplate reader. All experiments were conducted in triplicate. The concentration of PLL was established based on prior literature.
2.4. Animal models and experimental procedures
Six-week-old female athymic Balb/c nude mice were utilized for tumor xenograft experiments. The mice were housed in microisolator cages under sterile conditions and monitored for a minimum of one week before the initiation of the study to ensure optimal health. Environmental parameters, including temperature, lighting, and humidity, were centrally controlled. Before the experiments, all mice were anesthetized with 2 % isoflurane. In the orthotopic xenograft model, 2 × 106 MDA-MB-231 cells were implanted into the right mammary fat pad using a 29-gauge needle.
2.5. In vivo T2-weighted MR Imaging
T2-weighted MRI experiments were performed using a 3 T Philips MRI system. Anesthesia for the animals was induced with 3 % isoflurane and maintained at 2 % isoflurane in a gas mixture of 70 % N2O and 30 % O2. The respiration rate was continuously monitored using a small animal respiration pad, while the animal's body temperature was maintained using a warm-water tube integrated into the animal bed. The MR images were acquired using coronal T2-weighted rapid acquisitions with relaxation enhancement, characterized by the following parameters: repetition time of 3013 ms, echo time of 22.2 ms, slice thickness of 0.50 mm, acquisition matrix of 274 × 200, field of view of 300 × 180 mm, and a flip angle of 180.0°.
3. Results and Discussion
3.1. Characteristics of MNC
MNCs designed for use as T2 MR contrast agents were synthesized utilizing thermal decomposition methods. In this process, manganese iron in its inorganic state was integrated with Poly-L-Lysine (PLI) through an emulsion technique. Following this, any excess PLI was effectively removed via centrifugation, resulting in MNC with an optimized PLI ratio that is essential for their performance. As depicted in Fig. 1(A) and 1(B), transmission electron microscopy (TEM) was employed to rigorously assess both the size distribution and morphology of the MNC. The TEM analysis confirmed that the MNC exhibited an average size of 69 ± 27.4 nm, which is within the desirable range for biomedical applications. These results collectively suggest that the MNCs, engineered with a PLI matrix, are not only stably dispersed in aqueous solutions but also retain the capability to safely target and penetrate cancer cells in vivo. This characteristic enhances their potential utility as effective contrast agents in magnetic resonance imaging, thereby contributing to improved imaging resolution and accuracy in cancer diagnostics. Furthermore, the stability of the MNC in physiological conditions underscores their viability for future therapeutic applications, potentially facilitating targeted drug delivery systems in oncological therapies. Appropriately sized clusters could adequately avoid the reticuloendothelial system (RES), allowing them to stay longer in the blood. Additionally, the spherical morphology of the MNCs enhances their capacity to generate a stronger magnetic moment, which is crucial for their functionality. The surfaces of these MNCs are designed to be biocompatible, featuring charges that facilitate interaction with cellular structures. This biocompatibility, combined with their optimized shape, ensures that the MNCs can reliably and effectively target and reach diseased tissues. Such characteristics not only improve the targeting efficiency of the MNCs but also enhance their overall stability within biological environments. In Fig. 1(C) and the inset photograph, the MNCs showed a superparamagnetic nature without magnetic hysteresis. The saturation magnetization values of MNPs and MNC-1, MNC-2 at 1.0 T were 103.4, 84.4, 82.7 emu g-1Fe+Mn; MNC-1, MNC-2 were lower than those of MNPs owing to the presence of the organic polymeric layer. The enhanced magnetization efficiency of polymer-based magnetic nanoclusters, compared to individual magnetic nanoparticles, can be attributed to several physical and chemical characteristics. The polymer matrix strengthens the interactions between nanoparticles, facilitating the formation of aggregates that provide greater opportunities for generating higher magnetization during the magnetization process. Additionally, polymers offer the flexibility to control the size and shape of nanoparticles, optimizing their magnetic properties and enabling the achievement of desired magnetization efficiency for specific applications.
 |
Fig. 1.
(Color online) Transmission electron microscopy (TEM) images of (A) monodispersed Magnetic Nanoparticle (MNPs) and (B) Magnetic Cluster (MNC-2). The scale bar is 20 nm and 100 nm, respectively. (C) Magnetic hysteresis curves of MNPs and MNCs measured by using a vibration sample magnetometer. The inset shows the magnetic sensitivity of MNCs using the Nd-Fe-B magnet. |
The polymer matrix stabilizes the nanoparticles and increases their resistance to external stimuli, thereby enhancing magnetization efficiency. It also increases the surface area of the nanoparticles, augmenting the amount of surface available for magnetization reactions to occur. Furthermore, the polymer matrix provides the capability to modulate magnetic interactions, minimizing energy losses during the magnetization process. As the concentration of MNCs was increased (Fig. 2A), the T2 relaxivity (R2) of the MNC-1 and MNC-2 exhibited a linear enhancement, with T2 relaxivity coefficients measured at 224.5 and 402.1 mM¹s¹s¹ s⁻¹ (Fig. 2B). R2 is defined as the ratio of the iron (Fe) concentration to the inverse of the T2 relaxation time, and it is determined through the analysis of MR signal intensity across varying T2 relaxation durations. These findings suggest that the MNCs were stably suspended within the aqueous medium, the stability of the MNCs in solution, combined with their ability to facilitate the binding of therapeutic agents, positions them as promising candidates for future applications in diagnostic imaging and targeted therapy.
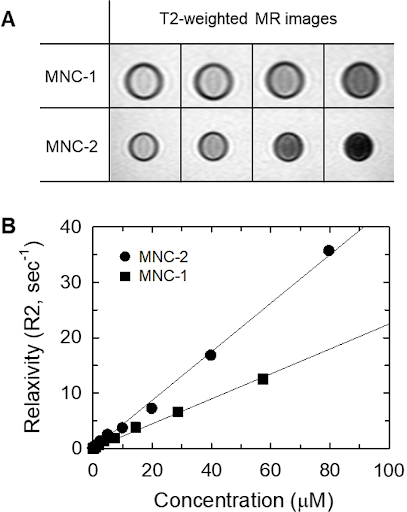 |
Fig. 2.
(A) T2-weighted MR images of MNCs in aqueous solution and (B) plots of relaxivity (R2) against MNC concentration. |
3.2. Cytotoxicity test for MNCs In Fig. 3(B), Cell viability assays were systematically conducted to evaluate the cytotoxicity of the MNC, with a focus on ensuring stable intracellular delivery. Employing the CCK-8 cell proliferation assay kit, meticulously assessed the effects of various concentrations of MNC on MDA-MB-231 cells to identify the concentration range that would not induce cellular damage. The results indicated that no significant detrimental effects on cell viability were observed starting from the 10-3 range, confirming the biocompatibility of the MNC at these concentrations. In addition, the internalization efficiency of the MNC was rigorously verified using transmission electron microscopy (TEM) in Fig. 3(A). The TEM analysis revealed that the MNC effectively formed endosomes within the cytoplasm of the cells. This observation is crucial as it demonstrates that the MNCs were able to enter the cells intact, without undergoing fragmentation or dispersion, which could compromise their functionality. This suggests that, when internalized as MNC rather than as single, uniform particles, there is potential for an even greater magnetic effect to be generated within the cells. And also, this finding provides compelling evidence that the MNC possesses the ability to achieve efficient and stable delivery into target cells. Such characteristics are essential for their potential applications in therapeutic interventions, particularly in the context of targeted drug delivery systems. The combination of low cytotoxicity and effective cellular uptake positions these MNCs as promising candidates for future research.  | Fig. 3. (Color online) (A) Transmission electron microscopy (TEM) images of MNCs in MDA-MB-231 cells (Triple Negative Breast Cancer Cells), scale bar is 100 nm. (B) is a Cell viability graph of MNCs. |
3.3. In vivo MRIIn vivo magnetic resonance imaging (MRI) was performed following the intravenous injection of MNC into the tail vein of mice (Fig. 4). After the administration of the MNC to anesthetized mice, imaging was systematically conducted at several time intervals: before administration, immediately post-injection, and subsequently at 1, 2, and 4 hours post-injection. Before the injection, no contrast enhancement was observed; however, immediately following the introduction of the MNC, a pronounced darkening of the central region of the tumor was evident, indicating significant and effective contrast enhancement (yellow arrow in Fig. 4). This initial enhancement suggests a successful uptake of the MNC by the tumor tissue. As time progressed, the observed contrast enhancement gradually diminished, which is considered an ideal phenomenon from the perspective of MRI contrast agents. This temporal reduction in signal intensity is indicative of the efficient clearance of the contrast agent, suggesting that the externally introduced MNC effectively navigates the biological endothelial barrier and demonstrates significant potential for renal excretion. Importantly, while some conventional contrast agents may exhibit favorable initial enhancement, they often have prolonged retention times within the body, which can pose potential risks, including toxicity or adverse effects. In contrast, MNCs demonstrate the ability to rapidly enhance contrast while minimizing retention time, thereby effectively mitigating associated risks. This rapid contrast enhancement underscores their capacity for swift accumulation in tumor tissues within a short timeframe, further enhancing their utility in targeted imaging applications. Such characteristics highlight the promising potential of MNCs not only for improved diagnostic imaging but also for future therapeutic interventions, as they may facilitate the targeted delivery of therapeutic agents directly to tumor sites. The ability to achieve both rapid visualization and effective treatment could significantly advance the field of magnetic contrast agents and enhance clinical outcomes in cancer.  Fig. 4. (Color online) In vivo T2 MR Images (coronal view) of tumor-bearing mice after injection of MNC. TR: 3013 ms, TE: 22.2 ms, Slice thickness: 0.5 mm).
4. ConclusionWe investigated the diagnostic capabilities of Manganese Ferrite (MnFe2O4) based MNC for triple-negative breast cancer (TNBC). TNBC is known for its high malignancy among breast cancer types and poses significant challenges for diagnosis due to the absence of specific biomarkers. Although conventional nanoparticle-based contrast agents exhibit some diagnostic potential, the MNC designed possesses a unique nanoball structure composed of multiple clustered particles. This configuration enhances the magnetic moment, resulting in significantly improved imaging capabilities within a shorter timeframe. The magnetic contrast agents are enveloped in a biocompatible coating of Poly-L-Lysine and Imidazole complex, ensuring excellent dispersion in aqueous solutions. This characteristic facilitates efficient targeting and delivery to the diseased tissues during intravenous administration. Moreover, the charge interactions between the MNCs and the densely packed cancer cells enable increased internalization into a greater number of cells within the tumor microenvironment. Future research will focus on loading these Poly-L-Lysine and Imidazole-based MNC with various therapeutic agents, aiming to develop multifunctional MNC capable of simultaneous diagnosis and treatment. This innovative approach holds great promise for enhancing the effectiveness of cancer management strategies, particularly in the context of aggressive malignancies like TNBC. ReferencesR. Dhabalia, S. V. Kashikar, P. Parihar, B. Nunna, S. S. Bothara and L. S. Reddy, CUREUS. 16, e68197 (2024). D. Santucci, E. Faiella, E. Cordelli, A. Calabrese, R. Landi, C. Felice, B. B. Zobel, R. F. Grasso, G. Iannello and P. Soda, Cancers. 13, 4635 (2021). [DOI]:10.3390/cancers13184635H. Lee, H. O. Kim, H. Y. Son, S. B. Lee, E. Jang, B. Kang, S. Haam, E. K. Lim and Y. M. Huh, J. Nanosci. Nanotechnol. 16, 12939 (2016). [DOI]:10.1166/jnn.2016.13797A. Shimauchi, S. A. Jansen, H. Abe, N. Jaskowiak, R. A. Schmidt and G. M. Newstead, Am. J. of Roentenology. 194, 1674 (2010). [DOI]:10.2214/AJR.09.3568
By Ph. D., M.D, Hwunjae Lee |




Comments
Post a Comment