Case Study: A 66-Year-Old Woman with Newly Developed Bilateral Upper Extremity Resting Tremors, Scans Without Evidence of Dopaminergic Deficit (SWEDD)
Scans Without Evidence of Dopaminergic Deficit (SWEDD)
1. Cause
and Etiology
SWEDD is not a disease
itself, but rather a diagnostic categorization
referring to patients who clinically
present with Parkinsonian symptoms (e.g., tremor, bradykinesia) but
whose dopamine transporter imaging
(DaTscan) does not show evidence
of dopaminergic neuron loss typically seen in Parkinson’s disease (PD)
or other Parkinsonian syndromes.
- Underlying
causes vary and include:
- Essential
tremor (ET)
- Dystonic
tremor
- Drug-induced
parkinsonism
- Functional
(psychogenic) movement disorders
- Vascular
parkinsonism
- Atypical
Parkinsonism without nigrostriatal degeneration
2. Pathophysiology
Unlike true Parkinson’s
disease, SWEDD patients do not show
degeneration of the substantia nigra or dopaminergic neuronal loss in the striatum.
- Normal DaTscan indicates:
- Intact
presynaptic dopaminergic terminals
- Absence
of nigrostriatal neurodegeneration
- Symptoms may arise from:
- Abnormal
cortical or cerebellar circuits (e.g., in dystonia or essential tremor)
- Non-organic
(functional) motor network dysregulation
3. Epidemiology
- Found
in 5–20% of patients
initially diagnosed with PD.
- More
common among:
- Females
- Younger
individuals at symptom onset
- SWEDD
cases do not progress like
Parkinson’s disease and tend to remain
stable or improve over time.
4. Clinical Presentation
SWEDD patients may
present with symptoms similar to early
Parkinson’s disease, including:
- Tremor
(often asymmetric and
resting)
- Bradykinesia
- Rigidity
However, SWEDD is often
distinguished by:
- Lack of true bradykinesia
with decrement
- No postural instability
- Poor or absent response to dopaminergic therapy (levodopa)
- Presence
of non-Parkinsonian signs:
- Fixed
postures (suggesting dystonia)
- Psychogenic
signs (e.g., distractibility, variability)
1.
Imaging Features
- DaTscan (Ioflupane I-123 SPECT):
Normal uptake in the striatum (putamen and caudate)
- Contrasts
with Parkinson’s disease, which shows decreased uptake, especially in the
posterior putamen
- MRI Brain: Usually normal; used to
exclude structural causes
- Functional imaging or PET scans
may be used in research, but not standard practice
6.
Treatment
Treatment depends on
the underlying cause rather than the SWEDD label itself:
- If essential tremor:
- Beta-blockers
(e.g., propranolol)
- Primidone
- If dystonic tremor:
- Anticholinergics
- Botulinum
toxin injections
- If drug-induced Parkinsonism:
- Withdrawal
of the causative agent
- If functional disorder:
- Multidisciplinary
approach (neurology, psychiatry, physiotherapy)
- Cognitive
behavioral therapy (CBT)
- Avoidance
of unnecessary dopamine therapy
Importantly, dopaminergic medications are usually ineffective
in SWEDD.
7. Prognosis
- Generally
favorable prognosis
- No
progression to typical Parkinson’s disease
- Some
patients may improve spontaneously or after targeted therapy
- Long-term
follow-up often leads to reclassification
of the diagnosis
🔍 Summary
|
Domain |
SWEDD Characteristics |
|
Definition |
Parkinsonism symptoms
with normal DaTscan |
|
Etiology |
Essential tremor,
dystonia, drug-induced, functional disorders |
|
Pathophysiology |
No dopaminergic
neurodegeneration |
|
Epidemiology |
5–20% of clinically
diagnosed PD patients |
|
Clinical Features |
Tremor, absent
response to levodopa, stable over time |
|
Imaging |
Normal striatal
uptake on DaTscan |
|
Treatment |
Based on the underlying
cause, not dopaminergic agents |
|
Prognosis |
Favorable, often
non-progressive |
====================================
Case Study: A 66-Year-Old Woman with Newly Developed Bilateral Upper Extremity Resting Tremors
Scans Without Evidence of Dopaminergic Deficit (SWEDD)
Clinical History and Imaging Findings
A 66-year-old woman presented with new-onset bilateral resting tremor in the upper extremities. Her recent medical history raised concerns for cognitive dysfunction.
Non-contrast axial T2-weighted and T1-weighted MRI brain images were obtained. These revealed no gross abnormalities in the basal ganglia, no significant cortical atrophy, and no evidence of hydrocephalus or intracranial mass.
Quiz 1:
What is the most prominent abnormal finding on MRI?
(1) Bilateral caudate atrophy
(2) Bilateral putaminal infarcts
(3) Significant cortical atrophy
(4) Hydrocephalus ex vacuo
(5) None of the above
Explanation: MRI revealed no significant structural abnormalities in the cortex or basal ganglia. There was no evidence of infarction, atrophy, or hydrocephalus.
Additional Imaging
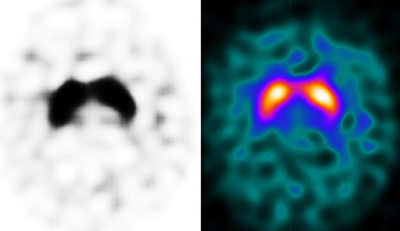
Dopamine transporter imaging was performed using I-123 Ioflupane single-photon emission computed tomography (DaTscan). The results demonstrated symmetric, comma-shaped radiotracer uptake in the striatal regions bilaterally, consistent with a normal dopaminergic profile. These findings are not supportive of Parkinson’s disease.
Quiz 2
(1) Symmetrical decreased radiotracer uptake in the posterior striatum
(2) Symmetrical, comma-shaped uptake near the central striatum
(3) Asymmetric decreased uptake in the right posterior striatum
Explanation: The DaTscan shows symmetric comma-shaped radiotracer uptake in both striata, consistent with a normal dopamine transporter distribution.
2. Which radiotracer is used in DaTscan imaging?
(1) Tc-99m MDP
(2) F-18 FDG
(3) In-111 oxine-labeled WBC
(4) I-123 Ioflupane
Explanation: DaTscan utilizes I-123 ioflupane, which binds to dopamine transporters in the striatum.
3. What is the principal photon energy of the DaTscan radiotracer?
(1) 140 keV
(2) 159 keV
(3) 173 keV
(4) 511 keV
Explanation: I-123 emits photons with a principal energy peak at 159 keV, suitable for gamma camera detection.
4. What is the half-life of I-123 Ioflupane?
(1) 109.7 minutes
(2) 6 hours
(3) 13.2 hours
(4) 2.8 days
Explanation: I-123 has a half-life of approximately 13.2 hours.
Differential Diagnosis
-
Parkinson’s disease
-
Corticobasal degeneration
-
Multiple system atrophy
-
Essential tremor
-
Scans without evidence of dopaminergic deficit (SWEDD)
Final Diagnosis:
Scans Without Evidence of Dopaminergic Deficit (SWEDD)
Discussion: SWEDD (Scans Without Evidence of Dopaminergic Deficit)
Pathophysiology and Epidemiology
SWEDD refers to a subgroup of patients initially diagnosed with Parkinsonism but who demonstrate normal dopamine transporter imaging. These individuals often present with isolated upper extremity resting or postural tremor and may not exhibit disease progression characteristic of idiopathic Parkinson’s disease. SWEDD is observed in approximately 10% of individuals initially diagnosed with Parkinson’s disease.
Clinical Features
-
Isolated upper extremity resting or postural tremor
-
Absence of clear Parkinsonian bradykinesia
-
Mild reduction in arm swing
-
Focal, mild dystonic posturing
Imaging Characteristics
DaTscan typically reveals symmetric, comma-shaped uptake in the striatum adjacent to the central line — a pattern that rules out presynaptic dopaminergic neuron loss.
Treatment and Prognosis
Management strategies include watchful waiting and symptomatic therapy. Some patients may receive levodopa-based medications, although the response is variable. The long-term prognosis of SWEDD patients tends to be more favorable than that of true Parkinson’s disease, with minimal clinical deterioration in many cases.
References (SCI-Level)
-
Morgante L, Espay AJ, Gunraj C, Lang AE. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? J Neurol Neurosurg Psychiatry. 2016;87(4):341–345. doi:10.1136/jnnp-2014-310256
-
Lee JW, Song YS, Kim H, Ku BD, Lee WW. Long-term follow-up study of SWEDD patients with mild Parkinsonian symptoms. BMJ Neurology Open. 2022;6(1):e000600. doi:10.1136/bmjno-2022-000600
-
Nicastro N, Garibotto V, Badoud S, Burkhard PR. Patients with scans without evidence of dopaminergic deficit: A 10-year retrospective study. Parkinsonism Relat Disord. 2016;24:79 84. doi:10.1016/j.parkreldis.2016.01.020
-
Pereira JB, Hall S, Jalakas M, et al. Frontotemporal lobe degeneration as the origin of scans without evidence of dopaminergic deficit. Front Neurol. 2018;9:335. doi:10.3389/fneur.2018.00335
-
Marek K, Jennings D, Seibyl J, et al. Scans without evidence of dopaminergic deficit (SWEDD) in early Parkinson’s disease: A longitudinal study. Neurology. 2005;64(12):2085–2090. doi:10.1212/01.WNL.0000165950.66163.03




Comments
Post a Comment