Cavernous Malformation: Comprehensive Overview
1. Causes (Etiology)
Cavernous malformations (CMs)
are vascular abnormalities of the central nervous system (CNS) and, less
frequently, other organs. They may arise sporadically or genetically.
- Sporadic CMs:
- Most common (~80% of cases).
- Typically isolated lesions without a family history.
- Often associated with adjacent developmental venous
anomalies (DVAs), which may contribute to their genesis via local venous
hypertension and hemorrhage.
- Familial CMs:
- Account for ~20% of cases.
- Inherited in an autosomal dominant manner
with incomplete penetrance and variable expressivity.
- Associated genes:
- CCM1 (KRIT1 gene, chromosome 7q21-22)
- CCM2 (MGC4607 gene, chromosome 7p13)
- CCM3 (PDCD10 gene, chromosome 3q26.1)
- Patients typically harbor multiple lesions
throughout life, and lesions may increase in number over time.
2. Pathogenesis
CMs are believed to form from:
- Abnormal angiogenesis: Loss of key proteins
(e.g., KRIT1, CCM2, PDCD10) disrupts endothelial cell-cell junctions,
leading to hyperpermeability, endothelial instability, and progressive
cavitation.
- Two-hit mechanism in familial cases:
- One inherited germline mutation, plus a second
somatic mutation ("second hit") leads to focal lesion
development.
- Hemodynamic factors: Especially in sporadic
cases associated with DVA, local changes in venous flow may cause
microhemorrhages and progressive cavernoma development.
Microscopically:
- Thin-walled, dilated capillary-like vascular
channels lined by a single endothelial layer without intervening brain
parenchyma.
- Absence of mature vessel wall elements (no smooth
muscle or elastic fibers).
- Often surrounded by hemosiderin deposits, indicating
prior microbleeds.
3. Pathophysiology
CMs disrupt CNS architecture
primarily through:
- Microhemorrhages:
- Recurrent minor bleeds cause hemosiderin
deposition, reactive gliosis, and chronic inflammation.
- Larger bleeds are rare but can be catastrophic if
in critical locations (e.g., brainstem).
- Mass Effect:
- Especially with large lesions or associated acute
hemorrhage.
- May lead to seizures, focal neurological deficits,
or increased intracranial pressure.
- Disruption of Blood-Brain Barrier (BBB):
- Hyperpermeability leads to local edema and
inflammatory responses, exacerbating clinical symptoms.
4. Epidemiology
- Prevalence:
- Estimated at 0.4%–0.8% in the general
population based on autopsy and MRI studies.
- Age:
- Diagnosed across all ages, but most symptomatic
presentations occur between 20–50 years.
- Sex:
- Slight female predominance reported in some
studies.
- Location:
- Supratentorial (80%)
- Infratentorial (15%)
- Brainstem (particularly dangerous) (5%)
- Spinal cord (rare)
- Natural history:
- Asymptomatic lesions may remain silent for life.
- Hemorrhage risk varies with prior hemorrhage
history, location (higher in the brainstem), and genetic background.
5. Clinical Features
CMs can be asymptomatic
(often incidentally found) or symptomatic with:
- Seizures (~40–70% of symptomatic
cases)
- Particularly in supratentorial lesions.
- Focal neurological deficits
- Depending on location and bleed (e.g., hemiparesis,
cranial nerve palsies in brainstem lesions).
- Headaches
- Nonspecific but may be present in symptomatic
individuals.
- Acute hemorrhage
- Less frequent than with arteriovenous malformations
(AVMs) but can cause sudden neurological deterioration.
- Spinal cord CMs
- Present with progressive myelopathy, radiculopathy,
or acute spinal cord syndrome after hemorrhage.
6. Imaging Findings
MRI is the modality of choice, with characteristic
features:
- T1-weighted imaging:
- Mixed signal intensity ("popcorn" or
"mulberry" appearance) due to hemorrhagic products at different
stages.
- T2-weighted imaging:
- Heterogeneous core with a hypointense hemosiderin
rim ("blooming effect").
- Gradient Echo (GRE) or Susceptibility-Weighted
Imaging (SWI):
- Highly sensitive for detection; lesions appear as
markedly hypointense foci due to susceptibility effects of hemosiderin.
- SWI can detect additional "occult"
lesions in familial cases.
- No significant contrast enhancement typically, unless
associated with recent hemorrhage or DVA.
CT scan:
- Limited utility.
- May show a hyperdense lesion if an acute hemorrhage is
present, but is often normal.
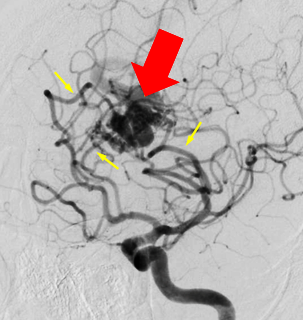
Angiography:
- CMs are typically angiographically occult (no
nidus, no early venous drainage).
7. Treatment
Management strategies depend
on clinical presentation, lesion location, and hemorrhagic history.
a. Conservative Management
- Asymptomatic or mildly symptomatic patients.
- Observation with serial MRI (frequency tailored
individually).
b. Surgical Resection
- Indications:
- Medically intractable seizures (lesions in
non-eloquent cortex).
- Progressive neurological deficits.
- Hemorrhagic lesions in accessible locations.
- Techniques:
- Microsurgical excision with minimal disruption of
adjacent brain tissue.
c. Radiosurgery
- Controversial.
- Some evidence supports the use for deep-seated
inoperable lesions (e.g., brainstem CMs).
- Efficacy is lower than in arteriovenous malformations
(AVMs).
- Delayed risk reduction in hemorrhage may take
several years.
d. Genetic Counseling
- Important for familial cases.
- Screening of family members with MRI may be
recommended.
8. Prognosis
Prognosis is highly
variable depending on factors such as:
- Location:
- Brainstem lesions have higher morbidity due to
critical anatomy.
- Prior hemorrhage:
- Annual hemorrhage risk:
- ~0.7% per lesion-year if no prior hemorrhage.
- Up to 4.5%–7% per lesion-year if prior symptomatic
hemorrhage.
- Seizure control:
- Good outcomes post-surgery in medically refractory
cases (seizure freedom rates 60–80%).
- Recurrence:
- Rare after complete resection in sporadic cases.
- New lesions can develop in familial cases.
Summary Table
|
Aspect |
Key Points |
|
Cause |
Sporadic or familial
(autosomal dominant) |
|
Pathogenesis |
Endothelial instability,
microhemorrhages, genetic mutations |
|
Pathophysiology |
Chronic bleeding, mass
effect, BBB disruption |
|
Epidemiology |
Prevalence 0.4–0.8%, slight
female predilection |
|
Clinical Features |
Seizures, focal deficits,
hemorrhage |
|
Imaging |
MRI (T1/T2/GRE/SWI); CT
limited; angiography negative |
|
Treatment |
Observation, surgery,
radiosurgery (selected) |
|
Prognosis |
Variable; worse with prior
bleeds, brainstem involvement |
============================
Case study: A 26-Year-Old Woman with Headache and Neurological Deficits
Cavernous Malformation
History and Imaging
-
A 26-year-old woman presented with daily headaches over the past month, accompanied by progressive gait instability and numbness on the right side.
-
A non-contrast head CT scan was performed.
Quiz 1:
-
Where is the most prominent abnormality located?
(1) Left tentorium cerebelli
(2) Left temporal lobe
(3) Left pons -
There is a mass effect on the fourth ventricle.
(1) True
(2) False
Additional Imaging:
-
A brain MRI without contrast was performed.
-
Selected images are shown below.
Quiz 2:
-
What is the most likely nature of this lesion?
(1) Benign
(2) Malignant -
This lesion is most commonly associated with:
(1) Developmental venous anomaly (DVA)
(2) Moyamoya disease
(3) Vein of Galen malformation -
Where is this lesion most commonly found?
(1) Supratentorial region
(2) Infratentorial region
Findings and Diagnosis
Findings:
CT imaging revealed a heterogeneous hyperdense lesion centered in the left occipital lobe and pons. The lesion appeared mass-like, with surrounding mild hypodensity and a localized mass effect leading to partial effacement of the fourth ventricle.
A heterogeneous, multilobulated lesion centered in the left occipital lobe and pons was observed on T1- and T2-weighted images. The lesion exhibited variable signal intensities, consistent with blood products of different ages. A hypointense rim was noted on T2-weighted images. A marked blooming artifact was observed on GRE sequences. Mild surrounding edema was present, and partial effacement of the fourth ventricle was noted.
Differential Diagnosis
-
Cavernous malformation
-
Cerebral amyloid angiopathy
-
Hemorrhagic metastasis
-
Hypertensive intracerebral hemorrhage
Diagnosis:
Cavernous malformation
Discussion
Cavernous Malformation
Pathophysiology
Cavernous malformations are characterized histopathologically by abnormally dilated, hyalinized capillaries without intervening normal brain parenchyma. Gliosis and hemosiderin deposition are typically found along the margins of these vascular channels. Loss of the integrity of the cerebral endothelial barrier eventually leads to symptoms and complications such as headache, seizures, and hemorrhagic stroke. Cavernous malformations can occur sporadically or be familial/inherited.
Epidemiology
Most cavernous malformations are considered congenital (either sporadic or autosomal dominant with incomplete penetrance), with a prevalence of up to 0.5%.
They account for approximately 5% to 15% of all central nervous system (CNS) vascular malformations.
Cavernous malformations are the second most common CNS vascular malformation, following developmental venous anomalies.
Clinical Features
The majority of patients with cavernous malformations are asymptomatic.
Patients with supratentorial lesions typically present with new-onset seizures and headaches.
Patients with infratentorial lesions often present with progressive neurological deficits.
Imaging Features
-
Distribution:
About 70–80% of cavernous malformations are located supratentorially.
Infratentorial cavernous malformations are associated with a higher risk of hemorrhage (~3.8%) compared to supratentorial lesions (~0.4%). -
CT Findings:
Cavernous malformations may be difficult to visualize on CT if they are small.
When visible, they appear as hyperdense areas resembling blood products or calcifications.
If a recent hemorrhage is present, the lesion may be more conspicuous and may be associated with surrounding edema. -
MRI Findings:
On MRI, cavernous malformations characteristically display a lobulated "popcorn-like" appearance with a peripheral hypointense hemosiderin rim on T2-weighted images. -
Zabramski Classification (1994):
This system categorizes cerebral cavernous malformations into four types based on conventional spin-echo and GRE MRI findings. Although useful for academic purposes, it is not routinely applied in clinical practice. One limitation is that it was developed before the widespread use of susceptibility-weighted imaging (SWI).-
Type I lesions:
Hyperintense center on T1WI; hypointense or hyperintense center on T2WI depending on the presence of intracellular/extracellular methemoglobin; reflects subacute hemorrhage. -
Type II lesions:
Mixed hyperintense and hypointense reticulated core on T2WI, surrounded by a hypointense rim; reflects chronic, evolving hemorrhages of various ages. -
Type III lesions:
Marked hypointensity and blooming on GRE, isointense or hypointense on T1WI; reflects chronic hemorrhage with residual hemosiderin within and around the lesion. -
Type IV lesions:
Small punctate hypointense lesions seen only on GRE or SWI, not well visualized on conventional T1/T2 imaging; represent minimal hemosiderin accumulation.
-
Cavernous malformations are often associated with developmental venous anomalies, which can also be identified on MRI.
Treatment
Management of cavernous malformations depends on clinical symptoms and lesion location, and includes conservative management, microsurgical resection, and stereotactic radiosurgery.
Surgical resection of brainstem cavernous malformations carries a higher risk of complications compared to lesions in other locations.
References
(1) Jain R, Robertson PL,
Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of
the brain. AJNR Am J Neuroradiol. 2005;26(5):1158-1162.
(2) Mouchtouris N, Chalouhi N,
Chitale A, et al. Management of cerebral cavernous malformations: From
diagnosis to treatment. ScientificWorldJournal. 2015;2015:808314.
(3) Phillips CM, Stamatovic SM,
Keep RF, Andjelkovic AV. Cerebral cavernous malformation pathogenesis:
Investigating lesion formation and progression with animal models. Int J Mol
Sci. 2022 Apr 30;23(9):5000.
(4) Wang KY, Idowu OR, Lin DDM.
Radiology and imaging for cavernous malformations. Handb Clin Neurol.
2017;143:249-266.






Comments
Post a Comment